Featuring
Patient Centricity & Collaboration Global Congress 2021 Europe
Current Trends & Strategies in Patient-centered Drug Discovery and Development
8th - 9th November 2021 - 2 Day Conference
London, UK
Venue
Hilton London Kensington
Address
179-199 Holland Park Ave, London W11 4UL
Phone
+44 (0) 207 193 3485
Global Congress 2021 Europe
Patient Centricity & Collaboration
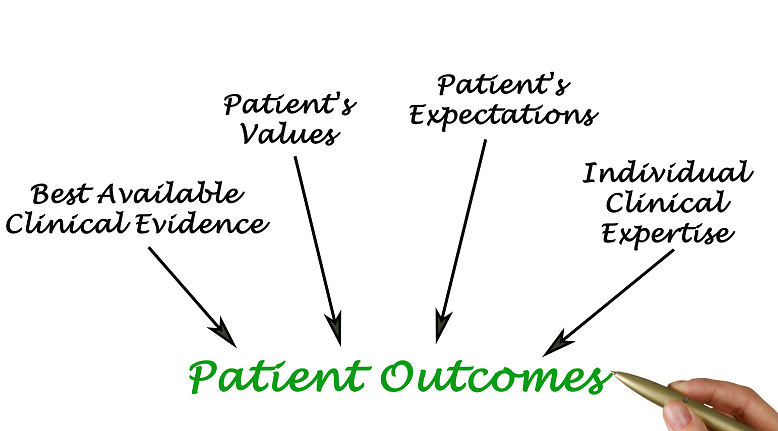
Patient-centricity is a vital aspect in the research and development of biopharmaceutical products, disease management, designing a treatment, clinical trial, or other health solutions. In order to create a patient-centric solution, one must truly embrace a collaborative endeavor with the patient and their caregivers. Establishing a patient-centric solution involves getting feedback from real patients and their loved ones, and making decisions based on their medical conditions, experiences, needs, perspectives, and priorities.


Featuring Key Industry Experts.
From a patient advocacy organization perspective, was a very informative meeting. Learned a lot from presentations and networking that will inform how we can add value to the advocacy-patient-drug developer dialogue.

Scientific Director, Research, Association for Frontotemporal Degeneration
I was very impressed with the format, the content was interesting and well done. I felt I learned a lot and was glad to be able to attend.

Business Development at Pharm-Olam, LLC